Few things vex a publicly traded company’s managers more than the prospect of admitting a mistake.
To acknowledge an error risks a stock selloff, bad publicity and possible litigation, as well as reduced executive pay and maybe even a few resignations. The alternative — covering up the blunder — could turn what is a professional embarrassment into a potential regulatory headache or even a criminal investigation.
The leaders of Penumbra, an extraordinarily successful Alameda, California–based manufacturer of neurovascular devices, have recently found themselves on the horns of this very dilemma.
Some surgeons have alleged that while they attempted to remove blood clots from patients’ brains, Penumbra’s newest catheter would occasionally break or fray, resulting in precious minutes spent addressing the fracture.
Penumbra’s answer baffled them: The company did not acknowledge the problem in any meaningful way; yet it did not fully deny it either.
On July 27, the company released a “Notification to Healthcare Providers,” reiterating its previous instructions for using the catheter, along with a warning against deploying it with non-Penumbra products.
But voluntary reports submitted to a Food and Drug Administration database, including entries from surgeons who have used the new catheter, paint a picture of a device whose safety problems Penumbra may be forced to address in more substantial terms than the July 27 release.
The launch of a modern medical legend
The device in question is the Jet 7 Reperfusion Catheter with Xtra Flex Technology, commonly known as the Jet 7 Xtra Flex.
Introduced with much fanfare at a key industry conference in July 2019, the Jet 7 Xtra Flex is an aspiration catheter designed for use in a procedure called a suction thrombectomy.
As shown in a brief Penumbra video clip, an aspiration catheter is a thin tube that can be inserted inside a person through an opening at the groin or wrist. Guiding the catheter to an arterial brain clot, a surgeon then uses suction to remove it. In this way, the artery’s blood flow can be restored.
In 2007 Penumbra received the first FDA approval to market an aspiration catheter designed to treat individuals who had experienced strokes. Following this, several clinical studies demonstrated the benefits of using an aspiration catheter for interventions after acute ischemic strokes, leading Penumbra to introduce several generations of its pioneering device.
The one-two punch of Penumbra’s being the first to market aspiration catheters with FDA approval and the rapid adoption of suction thrombectomy for the treatment of strokes has made the company’s devices ubiquitous in operating rooms.
From 2007 to 2018, some 80 percent of all the suction thrombectomies performed in the United States relied on Penumbra aspiration catheters, according to brokerage analysts.
Although much larger competitors like Stryker, Medtronic and Terumo introduced their own FDA-approved aspiration catheters in 2018, Penumbra still dominates the market: Penumbra aspiration catheters will play a leading role in nearly 65 percent of this year’s 45,000 to 50,000 suction thrombectomies in the U.S., according to projections by analysts during a Feb 25, 2020, Penumbra conference call.
From a business standpoint, Penumbra’s Jet 7 Xtra Flex catheter is a superstar, possibly accounting for at least 30 percent of the company’s sales last year. A research report that aggregated 2019 U.S. hospital purchasing orders put Jet 7 Xtra Flex sales to such facilities at $168 million, or 30.7 percent of the company’s total revenue. (An outside spokeswoman for Penumbra declined to provide specifics, citing the company’s policy of not disclosing precise sales figures.)
Penumbra’s success at improving its bottom line has propelled its share price ever higher, to its current $199.43, giving the 16-year-old company a gaudy $7.485 billion market capitalization.
MAUDE’s unflattering accounts
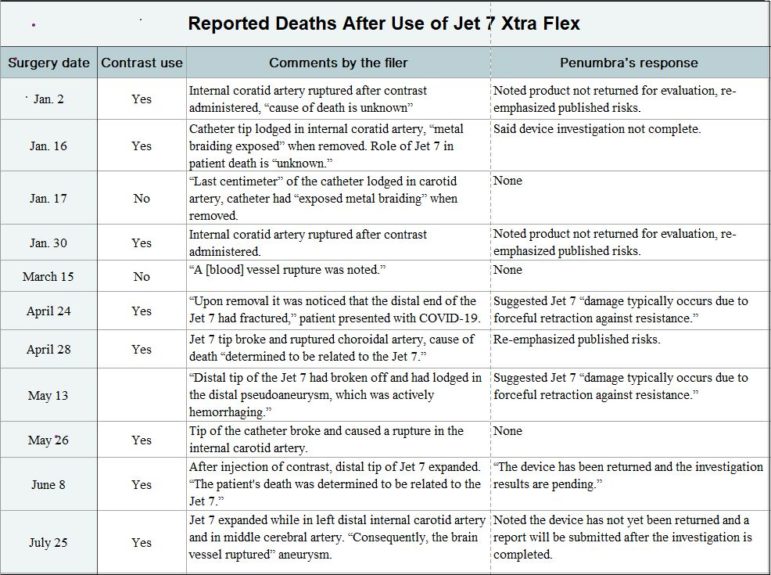
After Penumbra’s decade plus of success, the company now faces a very real quandary. The FDA’s Manufacturer and User Facility Device Experience (MAUDE) database lists 11 deaths that occurred after operations in January through the end of July that involved the Jet 7 Xtra Flex. The MAUDE database has one such report of a death after an operation last year; Penumbra rolled out the new catheter commercially in mid-2019.
MAUDE is an informal, voluntary reporting system for public tracking of adverse events like injuries and deaths involving any piece of medical equipment approved for use in the U.S. A wide array of individuals, including medical professionals, family members of people operated on, as well as company representatives, can submit entries to MAUDE, so the reports vary in their amount of detail. And unlike a clinical study or an autopsy, MAUDE entries are not necessarily recorded in a scientific or medical fashion.
(In this manner, MAUDE resembles another database, the FDA Adverse Event Reporting System, which is a repository of adverse pharmaceutical event reports. The Foundation for Financial Journalism used FAERS data to reveal a pattern of significant numbers of fatal drug reactions from products sold by Corcept Therapeutics, Acadia Pharmaceuticals and Insys Therapeutics.)
Despite their limitations, MAUDE records are certainly valuable for anyone hoping to detect a possible trend.
All 12 MAUDE entries detailing deaths after 2019 and 2020 operations that involved the Jet 7 Xtra Flex catheter mentioned the device’s distal tip suddenly expanding or fracturing. And eight reports noted at least one arterial rupture – with many of the ruptures cited as occurring in the internal carotid artery, which supplies blood to the brain and eyes. (One reported death, after an April 24 operation, involved a person with COVID-19, a condition that may have complicated that individual’s outcome.)
When asked about the 11 deaths from January through the end of July listed in the MAUDE database, Penumbra’s marketing chief Gita Barry acknowledged that the company is aware of the reported deaths, stating, “Penumbra filed medical device reports for all adverse events with the FDA which are reflected in the MAUDE database.”
Barry added, “Following our investigation into the reports, we worked diligently to communicate directly with physicians.”
A formal investigation is not required before someone submits a MAUDE entry. And only two of the 11 reports concerning 2020 procedures ― entries about surgeries on June 8 and April 28 ― overtly alleged that the fatalities were linked to Jet 7 Xtra Flex problems. Three other entries categorized the relationship between the Penumbra catheter and a death as “unknown.”
Nine MAUDE entries describing 2020 operations, however, claimed the Jet 7 Xtra Flex either fractured or expanded shortly after a surgeon began a cerebral angiography. In the latter type of procedure, a catheter is injected with an iodine contrast dye to make the artery ― and the clot ― visible on X-rays. Angiography has been a standard component of clot-removal surgery for years.
To be fair, the Jet 7 Xtra Flex’s label — rendered in small print — clearly warns against injecting contrast dye into the catheter and also recommends it not be used with non-Penumbra products. Plus, anyone undergoing neurovascular surgery after having a stroke or an aneurysm is indeed at a heightened risk for having numerous complications, including death.
But Penumbra’s insistence that its catheters are safer to use when paired with other Penumbra products flies in the face of current medical practice, according to three neurovascular surgeons interviewed by the Foundation for Financial Journalism. (These doctors spoke on the condition of anonymity due to their employers’ prohibitions on talking with the press, and one surgeon had even received speaking fees from Penumbra.) All three doctors said use of Penumbra’s aspiration catheters with non-Penumbra devices is a “nearly universal practice” of surgeons performing thrombectomies.
“I like the Jet 7 [Xtra Flex catheter] well enough,” said one of the three physicians. “But I don’t like nearly anything else [Penumbra] has out. So I use a microcatheter, stents and coils from other companies.”
All 11 MAUDE reports detailing 2020 procedures referred to this mix-and-match practice. The entry about a March 15 operation, for example, noted the doctor had used a Jet 7 Xtra Flex alongside “a non-Penumbra microcatheter and non-Penumbra revascularization device.”
Since surgeons who treat strokes tend to live by the creed “time is brain” (meaning speed is of the essence when trying to prevent long-term neurological impairment), they are unlikely to want to swap out a Jet 7 Xtra Flex (after performing a suction thrombectomy) for another catheter to do the angiography.
Penumbra’s Barry, however, insisted that injecting contrast dye into an aspiration catheter is exactly what doctors should avoid doing. “Reperfusion catheters are designed for the removal of stroke-causing clots by aspirating or suctioning the clot out of the arteries in the brain, and not for contrast injection,” Barry said. Using the aspiration catheter for the dye task runs the risk of reintroducing a clot into the brain’s arteries, which could prompt an additional stroke, she added.
Two of the three surgeons interviewed by the Foundation for Financial Journalism said they do change catheters when performing an angiography during a suction thrombectomy. “But there is an expectation that the [aspiration] catheter can withstand an injection,” one doctor noted, adding that at times using the same catheter for both tasks is clinically valuable.
New wrinkles in the competitive landscape
Maybe the most problematic aspect for Penumbra concerning MAUDE lies with what is not described there: deaths related to competitors’ aspiration catheters. MAUDE carries no entries (filed through July 31) for Medtronic’s React 68 and 71, Stryker’s AXS Vecta 71 and Terumo’s Sofia Plus aspiration catheters.
And a key Penumbra competitor has seized on Jet 7 Xtra Flex’s troubles as a marketing opportunity. Medtronic has been running a digital advertisement on its neurovascular unit’s LinkedIn page, touting its aspiration catheter’s ability to “manually deliver contrast injections.” The logic behind Medtronic’s running the ad is simple: While few surgeons probably spend much time on LinkedIn, Penumbra sales staffers — unaccustomed to marketing a device whose safety profile is being questioned — might be weighing their career options, and some of them may be tempted to defect.
On Aug. 27, Piper Sandler research analyst Matt O’Brien hosted a virtual “fireside chat” for his firm’s money management clients with Stacey Pugh, general manager of Medtronic’s neurovascular unit. Boldly assessing her company’s performance this past summer, she said, “Candidly, we’ve been very pleased with our average daily sales [of aspiration catheters] since that notice went out,” referring to Penumbra’s July 27 missive.
Medtronic’s sales in Japan are being helped by what Pugh described as a “voluntary recall” of Penumbra’s Jet 7 Xtra Flex, she said. “There’s a void in the marketing of [Penumbra’s] product in Japan” that has created a built-in sales growth opportunity for Medtronic, she asserted. (Pugh’s candor may have been bolstered by the Piper Sandler event’s closure to the press.)
Asked about Pugh’s description of a “voluntary recall” in Japan, Penumbra’s Barry replied, “No, this is not true. The product has not been recalled in Japan.” Penumbra’s Japanese distributor “paused sales” while her company updated the Jet 7 Xtra Flex’s “instructions for use,” she said. After Japanese regulators approve the catheter’s new instructions, the device will be restocked, Barry claimed.
The Japanese market accounted for 7.8 percent of Penumbra’s sales in 2019, or $42.5 million, according to its annual report.